Abstract
Introduction: High dose therapy with Allogeneic Haematopoietic Stem Cell Transplantation (AlloHSCT) has traditionally been performed as an inpatient procedure. However, with improvements in supportive care and patient selection it is possible to safely deliver conditioning chemotherapy in an outpatient setting (Subirà M et al., 2003). While deemed an "outpatient procedure" this method is currently delivered on large day units which requires the patient to attend daily, often only spending the night at home.
To reduce these daily visits The Royal Melbourne Hospital (RMH) Bone Marrow Transplant department in collaboration with the Hospital in the Home (HIH) department developed an innovative program to safely deliver Fludarabine chemotherapy as part of conditioning for AlloHSCT in the patient's home. This process involved a daily patient review by a HIH Doctor as well as twice daily visits by a HIH nurse to administer chemotherapy and supportive care in the comfort of the patient's home. Here we report on the safety outcomes of HIH AlloHSCT vs inpatient (IP) care, specifically complications and outcomes.
Methods: A retrospective case note audit identified 395 consecutive AlloHSCT patients who received Fludarabine based conditioning between 2011 and 2017 at the RMH. Of these 130 received Fludarabine as part of the HIH Program and 265 as a traditional IP.
Results: Of those treated as HIH patients, median age was 51 years (range 18-69). 59% patients were male (n=77) and 41% Female (n=53). Underlying disease groups included Leukaemia (n=53; 40%), Lymphoma (n=35; 27%), Myelodysplatic/Myeloproliferative (n=28; 21%), Myeloma (n=11; 9%) and Non-malignant (n=3; 3%). 2% of patients had myeloablative conditioning (n=2) and 98% received Reduced Intensity Conditioning (n=128). 57% (n=73) received stem cells from a sibling (Sib), 41% (n=54) from Voluntary Unrelated Donors (VUD) and 2% (n=3) from Umbilical Cord Donors.
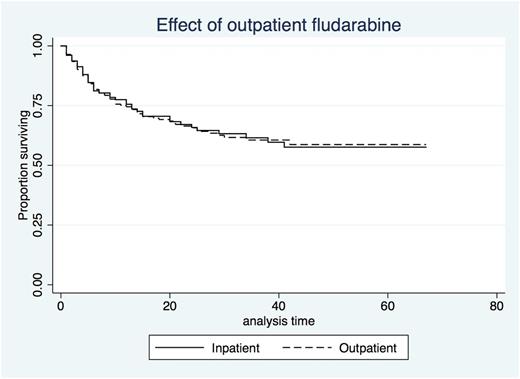
Median total Length of Stay (LOS) for HIH patients was 27 days (Range 10-97) with a median of 5 days (range 2-8) at home as part of this. The LOS for those receiving fludarabine as an IP was not significantly different at 26 days (range 5-174; p=0.209). Total number of IP Bed days saved through the HIH program was 682. There was no significant difference in median time to neutrophil engraftment (HIH 20 vs IP 17 days; p =0.95) or platelet engraftment (HIH 23 vs IP 20 days; p=0.99). Having chemotherapy as a HIH patient had no effect on outcome (HR 1.01). There is no difference in Overall Survival (OS) between the two groups (HIH 17 vs IP 16 months - see Figure 1) while Median OS was not reached.
Of the 130 patients who were treated in the HIH Program only 11 had an unplanned admission before Day 0 of their AlloHSCT. Reasons for readmission included suspected infection (n=8; only one culture positive), vasovagal (n=1) and anxiety (n=2). Of note, four of these admissions arose in the first three months of the program with the other seven readmissions occurring over the following six years, with a readmission rate of approximately 5% (7/126 patients).
Conclusion: The HIH delivery of chemotherapy and supportive care as part of conditioning for AlloHSCT in the patient's home is both safe and effective. It resulted in a median of five bed days saved per patient (Total number of bed days saved = 682), allowing the department to increase bed capacity without the costs associated with building a new ward. Additionally, the risk of complications was low (approximately 5%) and those that did occur were safely managed.
Figure 1 - Comparison of Survival Between HIH and IP AlloHSCT Patients
Routledge: Celgene: Honoraria; Gilead: Honoraria; Bristol-Myers Squibb: Honoraria. Ritchie: Amgen Inc.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.